Questions and answers on current good manufacturing practices. Questions and answers on current good manufacturing practicesrecords and reports. Controlled processes will achieve fairly consistent waste amounts batchtobatch. Electronic records;
Electronic batch record system dassault systèmes biovia. Transforming batch records through paper free and automated execution. Biovia ebr is an out of the box procedure execution and electronic batch record software system designed to provide a low cost of ownership electronic environment to efficiently and compliantly document the execution of any procedure or recipe.
Healthcare Kpi List
Making the move to electronic batch records pharmaceutical. From a compliance standpoint, an electronic batch record solution helps an organization meet 21 code of federal regulations (cfr) part 11, which defines the manner in which fda accepts electronic records and electronic signatures (1). By putting electronic batch records in place, an organization. Using electronic batch records to their full potential. Quality assurance (qa) teams can use an ebr’s reviewbyexception functionality to spot deviations much faster than scanning every record line by line to find them. This can speed up documentation review times and reduce how long a product must sit in a warehouse waiting for release. It also can improve batch accuracy and consistency. Healthcare records. Healthcare records govtsearches. Search for health records online at directhit. Electronic health records centers for medicare & medicaid. Find health record. Get high level results! Two case studies electronic batch record how to reduce. Systems and tools for batch record evaluation the use of checklists batch record review sop optimisation case studiesthis course is electronic batch record how to reduce review time efficient batch record review 2627 june 2014, budapest, hungary two case studies electronic batch record how to reduce review time. Health record selected results find health record. Healthwebsearch.Msn has been visited by 1m+ users in the past month. Health record video results. Find health record if you are looking now.
Cfr code of federal regulations title 21. (B) your batch production record must include complete information relating to the production and control of each batch; (c) your batch production record must accurately follow the appropriate master manufacturing record and you must perform each step in the production of the batch; and (d) you must make and keep batch production records in. Montgomery county health department our mission to promote, protect and improve the health and prosperity of people in tennessee naloxone training, certification, and free kit available every 3rd wednesday of each month, from 530p.M. 600p.M. At civic hall in the veteran's plaza. Improving operations through electronic batch records. Ages and sequences batch records, collects and consolidates all batch data and generates facility performance metrics, graphs and charts. † An electronic batch records module that provides routing, review and approval of paperless manufacturing records for current good manufacturing practices (cgmp) facilities with complete product and activity. Electronic batch record system dassault systèmes biovia. Transforming batch records through paper free and automated execution. Biovia ebr is an out of the box procedure execution and electronic batch record software system designed to provide a low cost of ownership electronic environment to efficiently and compliantly document the execution of any procedure or recipe. Health record definition of health record by medical dictionary. Everymanbusiness has been visited by 100k+ users in the past month.
Montgomery county health department. Get more related info visit us now discover more results. Electronic batch records mastercontrol. Electronic batch records demonstrate accountability by providing proof of proper handling of every significant step in the production of each batch of a product. In addition, fda regulated companies that execute and document batch records electronically must comply with the fda's 21 cfr part 11 requirements and good manufacturing practices. Dermatology electronic records find top results. Directhit has been visited by 1m+ users in the past month. The terms medical record, health record, and medical chart are used somewhat interchangeably to describe the systematic documentation of a single patient's medical history and care across time within one particular health care provider's jurisdiction. Who should review batch records? The mistake in gmp. It is a requirement of european union gmp (eu gmp) that batch records are reviewed prior to batch release. But european union gmp is no longer clear about who should do this, and with recent updates to eu gmp chapter 2 on personnel it looks like a big omission has occurred. In eu gmp three key personnel are named. Electronic batch records in pharmaceuticals pharmaceutical. In 1997, electronic batch records in pharmaceuticals were accepted by fda after ebr systems showed compliance with fda regulations. Pharmaceutical companies are obliged to adopt the use of automated electronic batch records to ensure they comply with the current good manufacturing practice regulations set by fda. Why batch manufacturing records are so important according to. Batch manufacturing record is like a back bone in the current good manufacturing practices (cgmp), and very essential to stay compliance. Recent posts the all about electronic batch recording software,mpcr and bpcr.
Patient Portal Integris
Dermatology electronic records find top results. Only you or your personal representative has the right to access your records. A health care provider or health plan may send copies of your records to another provider or health plan only as needed for treatment or payment or with your permission. Your medical records hhs.Gov. Find fast answers for your question with govtsearches today! Health records online now directhit. The service is an online service designed to allow you to communicate with your medical care providers. You can send secure messages to your provider, request an appointment, check on your lab results, view your health record, request a prescription refill, complete registration and health information forms, and read patient education. Electronic batch record (ebr) software systems mastercontrol. Mastercontrol’s electronic batch records system contains everything you need to create complete and compliant batch records. With its simple configuration and robust functionality, mastercontrol ebr meets the unique and changing needs of your company now and in the future. Electronic batch record allinone gmp software. Instantgmp™ pro is a gmp software that utilizes electronic batch records that organize and manage batch documentation, as well as reinforce good manufacturing practices. The software guides users through the work flows required by part 211 for pharmaceutical and biotech products and part 111 for dietary supplements. Instantgmp™ pro is an. Log in myhealthrecord. Govtsearches has been visited by 100k+ users in the past month.
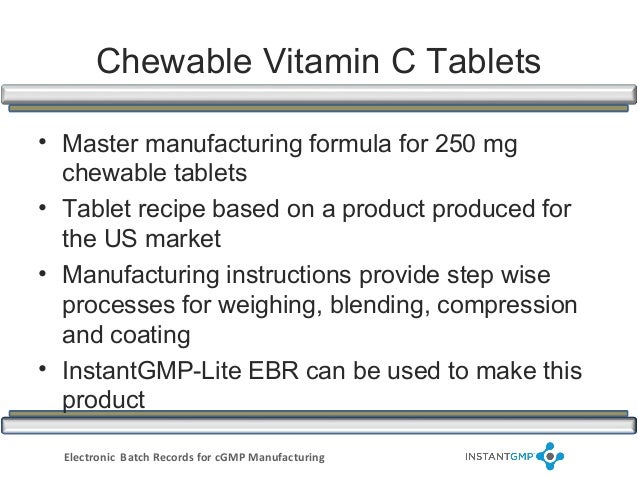
Master batch records werum it solutions gmbh. Master batch records allows the user to set up libraries with standardized, reusable building blocks which can then be used to create a master batch record. These building blocks are subject to version management. Master batch records are defined and displayed using a graphical design tool. Healthcare records. Healthcare records govtsearches. Health record as used in the uk, a health record is a collection of clinical information pertaining to a patient's physical and mental health, compiled from different sources. Medical record wikipedia. Internetcorkboard has been visited by 1m+ users in the past month. Documentation and records harmonized gmp requirements. Batch records these documents are typically used and completed by the manufacturing department. Batch records provide stepbystep instructions for productionrelated tasks and activities, besides including areas on the batch record itself for documenting such tasks. An electronic health record (ehr) is an electronic version of a patients medical history, that is maintained by the provider over time, and may include all of the key administrative clinical data relevant to that persons care under a particular provider, including demographics, progress notes, problems, medications, vital signs, past medical history. Questions and answers on current good manufacturing practices. Questions and answers on current good manufacturing practicesrecords and reports. Controlled processes will achieve fairly consistent waste amounts batchtobatch. Electronic records;
Health Informatics Diploma
Health records online now directhit. Also try. More health record videos. Electronic batch record system dassault systèmes biovia. Transforming batch records through paper free and automated execution. Biovia ebr is an out of the box procedure execution and electronic batch record software system designed to provide a low cost of ownership electronic environment to efficiently and compliantly document the execution of any procedure or recipe. Electronic batch record allinone gmp software. Instantgmp™ pro is a gmp software that utilizes electronic batch records that organize and manage batch documentation, as well as reinforce good manufacturing practices. The software guides users through the work flows required by part 211 for pharmaceutical and biotech products and part 111 for dietary supplements. Instantgmp™ pro is an. Electronic batch records archives instantgmp, inc.. Cary, nc instantgmp inc., Creator of an electronic batch record system, instantgmp™ mes 3.0, announces the release of a new feature that connects equipment on the production floor with its cloudbased software. Using electronic batch records (ebr) to optimize. Electronic batch records. Producing the mountains of paperwork required for production batch records is a timeconsuming and errorprone process that often extends release time. Electronic batch record systems demonstrate accountability by providing proof of proper handling for every step in the production of each batch of a drug product. Instantgmp, inc. Affordable allinone manufacturing. Affordable allinone manufacturing & quality software. Benefits of instantgmp™ developed by quality and regulatory experts, our software simplifies batch & quality management to provide your company with a gameplan for success. Directhit has been visited by 1m+ users in the past month.